The Clinical Validation Difference
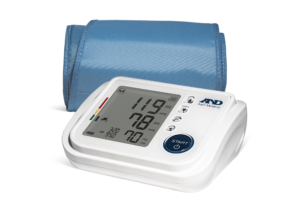
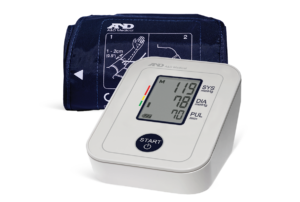
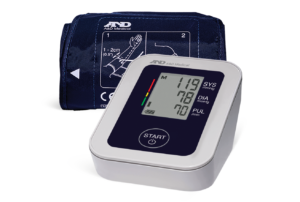
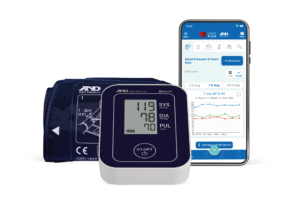
For over 40 years, A&D Medical has been a world leader in precise and accurate blood pressure monitors. A&D Medical always verifies their blood pressure monitors through a process called clinical validation. This means that our products are tested by an independent party, use industry-standard protocols, and are published in a peer-reviewed journal.
Until recently there wasn’t an organization in the United States that verified any of these results and it was difficult for a buyer to determine a blood pressure monitor’s accuracy. Why is accuracy so important? Because accurate measurement of blood pressure is critical for the diagnosis and management of hypertension.
The American Medical Association (AMA) recognized this gap in the blood pressure category and realized a standardized validation process would help eliminate confusion surrounding a product’s accuracy. The AMA developed clinical accuracy criteria with a group of subject matter experts.
The National Opinion Research Center (NORC) at the University of Chicago manages a committee of physician experts, who independently review and determine the accuracy of inclusion of the US BP Validated Device Listing (VDL).
A&D Medical is proud to be one of the first manufacturers to be included in the VDL and we continue to add products. It is our commitment to make sure our customers can trust their blood pressure measurements whether in the home, community, or clinical setting.